New Predictive Low Glucose Suspend Feature to Launch with Dexcom G6 CGM System Integration
June 21, 2018 04:05 PM Eastern Daylight Time
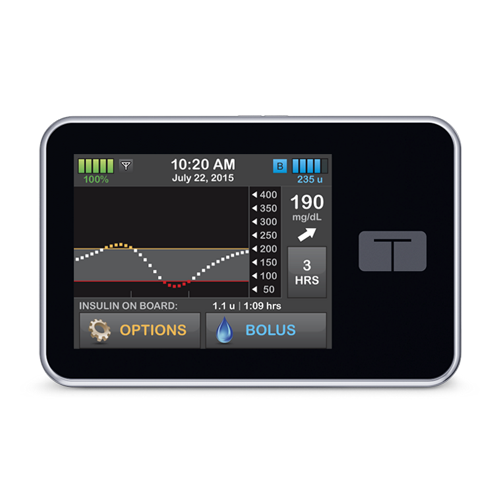
SAN DIEGO–(BUSINESS WIRE)–Tandem Diabetes Care®, Inc. (NASDAQ: TNDM), a medical device company and manufacturer of the only touchscreen insulin pumps available in the United States, today announced U.S. Food and Drug Administration (FDA) approval of the t:slim X2™ Insulin Pump with Basal-IQ™ technology, a predictive low glucose suspend (PLGS) feature designed to help reduce the frequency and duration of low glucose events (hypoglycemia). This is the first automated insulin delivery system approved for use by children as young as 6 years old, and the first insulin pump designated as compatible with integrated continuous glucose monitoring (iCGM) devices. The Company plans to launch its new product with Dexcom G6® continuous glucose monitoring (CGM) integration, which requires no fingersticks for calibration or diabetes treatment decisions and was the first CGM device to receive the iCGM designation from the FDA earlier this year.1,2,3 Tandem expects the t:slim X2 Pump with Basal-IQ technology to be available in August 2018, and all in-warranty t:slim X2 users in the United States will have the option to add the new feature free of charge via remote software update.4
Læs hele pressemeddelelsen her.